The association between vancomycin trough concentrations and nephrotoxicity in the paediatric intensive care unit
Main Article Content
Abstract
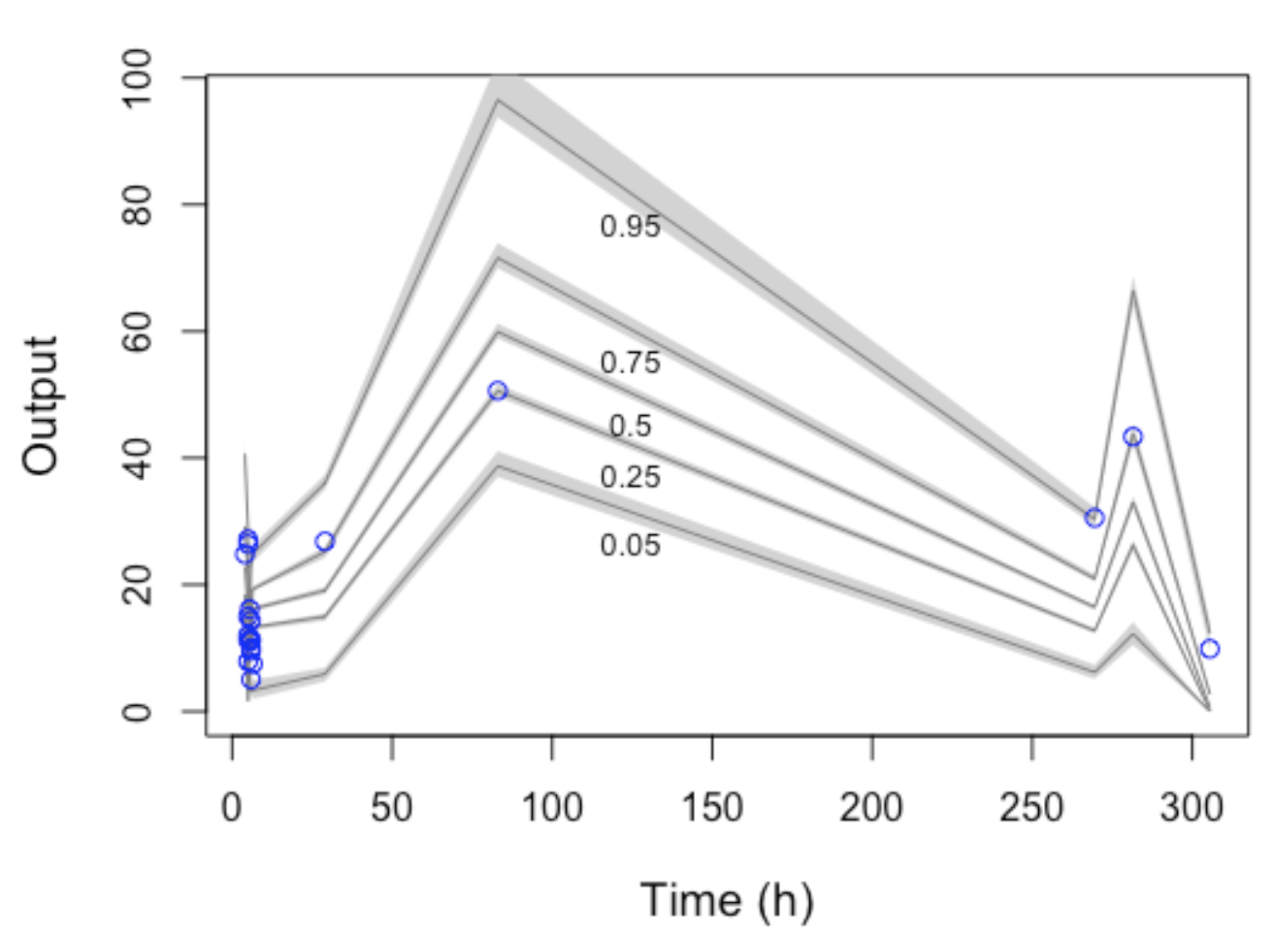
Objective: To analyze and describe the pharmacokinetic aspects of vancomycin usage in a cohort of critically ill children and to construct a pharmacokinetic model for this population. Method: We conducted an observational study in a pediatric intensive care unit from September 2017 to March 2019. Children receiving vancomycin with at least one serum measurement were included. Variables with a p-value lower than 0.2 in univariate analysis, and biologically plausible for inducing nephrotoxicity and not correlated with other predictors, were incorporated into logistic regression. Additionally, pharmacokinetic modeling was performed using the PMETRICS® package for patients with creatinine clearance (CLCR) > 30 mL/min. Result: The study included 70 children, with an average vancomycin dose of 60 mg/kg/day. Only eleven children achieved vancomycin levels within the target range (15-20 mg/L). No significant differences in doses/mg/kg/day were observed among children above, within, or below the vancomycin target range. In the multivariate model, children above the recommended serum range had an odds ratio of 4.6 [95% CI 1.4 – 17.2] for nephrotoxicity. A pharmacokinetic model was proposed using data from 15 children, estimating PK parameters for CLCR and V as 0.94 L/h and 5.71 L, respectively. Conclusion: Nephrotoxicity was associated with vancomycin plasma concentrations equal to or exceeding 15 mg/L. The developed model enhanced understanding of the drug’s behavior within this population, potentially aiding clinical practice in dose calculations and estimation of the area under the curve – a recommended parameter for vancomycin monitoring.
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors maintain copyright and grant the HSJ the right to first publication. From 2024, the publications wiil be licensed under Attribution 4.0 International 
 , allowing their sharing, recognizing the authorship and initial publication in this journal.
, allowing their sharing, recognizing the authorship and initial publication in this journal.
Authors are authorized to assume additional contracts separately for the non-exclusive distribution of the version of the work published in this journal (e.g., publishing in an institutional repository or as a book chapter), with acknowledgment of authorship and initial publication in this journal.
Authors are encouraged to publish and distribute their work online (e.g., in institutional repositories or on their personal page) at any point after the editorial process.
Also, the AUTHOR is informed and consents that the HSJ can incorporate his article into existing or future scientific databases and indexers, under the conditions defined by the latter at all times, which will involve, at least, the possibility that the holders of these databases can perform the following actions on the article.
References
2. Araujo da Silva AR, Jaszkowski E, Schober T, von Both U, Meyer-Buehn M, Marques AF, et al. Patterns of antimicrobial consumption in neonatal and pediatric intensive care units in Germany and Brazil. Eur J Clin Microbiol Infect Dis. 2020;39(2):249-55. http://doi.org/10.1007/s10096-019-03714-9. PMid:31673879.
3. Sridharan K, Abbasi MY, Mulubwa M. Population pharmacokinetics and dose optimization of vancomycin in critically Ill children. Eur J Drug Metab Pharmacokinet. 2021;46(4):539-46. http://doi.org/10.1007/s13318-021-00695-z. PMid:34156647.
4. Cao L, Li Z, Zhang P, Yong S. Relationship between vancomycin trough serum concentrations and clinical outcomes in children: a systematic review and meta-analysis. Antimicrob Agents Chemother. 2022;66(8):e00138-22. http://doi.org/10.1128/aac.00138-22. PMid:35862741.
5. He N, Su S, Ye Z, Du G, He B, Li D, et al. Evidence-based Guideline for Therapeutic Drug Monitoring of Vancomycin: 2020 Update by the Division of Therapeutic Drug Monitoring, Chinese Pharmacological Society. Clin Infect Dis. 2020;71(Suppl 4):S363- 71. http://doi.org/10.1093/cid/ciaa1536. PMid:33367582.
6. Filippone EJ, Kraft WK, Farber JL. The nephrotoxicity of vancomycin. Clin Pharmacol Ther. 2017;102(3):459-69. http:// doi.org/10.1002/cpt.726. PMid:28474732.
7. Shah S, Barton G, Fischer A. Pharmacokinetic considerations and dosing strategies of antibiotics in the critically ill patient. J Intensive Care Soc. 2015;16(2):147-53. http://doi.org/10.1177/1751143714564816. PMid:28979397.
8. McNeil JC, Kaplan SL. Vancomycin therapeutic drug monitoring in children: new recommendations, similar challenges. J Pediatr Pharmacol Ther. 2020;25(6):472-5. http://doi.org/10.5863/1551-6776-25.6.472. PMid:32839650.
9. Lee BV, Fong G, Bolaris M, Neely M, Minejima E, Kang A, et al. Cost-benefit analysis comparing trough, two-level AUC and Bayesian AUC dosing for vancomycin. Clin Microbiol Infect. 2021;27(9):1346.e1-7. http://doi.org/10.1016/j.cmi.2020.11.008. PMid:33221430.
10. Tsutsuura M, Moriyama H, Kojima N, Mizukami Y, Tashiro S, Osa S, et al. The monitoring of vancomycin: a systematic review and meta-analyses of area under the concentration-time curve-guided dosing and trough-guided dosing. BMC Infect Dis. 2021;21(1):153. http://doi.org/10.1186/s12879-021-05858-6. PMid:33549035.
11. Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835- 64. http://doi.org/10.1093/ajhp/zxaa036. PMid:32191793.
12. Zamoner W, Prado IRS, Balbi AL, Ponce D. Vancomycin dosing, monitoring and toxicity: Critical review of the clinical practice. Clin Exp Pharmacol Physiol. 2019;46(4):292-301. http://doi.org/10.1111/1440-1681.13066. PMid:30623980.
13. Chung E, Sen J, Patel P, Seto W. Population pharmacokinetic models of vancomycin in paediatric patients: a systematic review. Clin Pharmacokinet. 2021;60(8):985-1001. http://doi.org/10.1007/s40262-021-01027-9. PMid:34002357.
14. World Health Organization [Internet]. Geneva: WHO; 2023 [cited 2023 Aug 7]. Available from: https://www.who.int
15. Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering RC Jr, Craig WA, Billeter M, et al. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the Infectious Diseases Society of America, the American Society of Health- System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis. 2009;49(3):325-7. http://doi.org/10.1086/600877. PMid:19569969.
16. UpToDate. Lexicomp® Drug Interactions [Internet]. 2023 [cited 2023 Aug 10]. Available from: https://www.uptodate.com/drug-interactions/?source=responsive_home#di-druglist
17. Laboratory of Applied Pharmacokinetics and Bioinformatics. Pmetrics [Internet]. 2023 [cited 2023 Aug 10]. Available from: https://www.lapk.org/software.php
18. Feiten HDS, Okumura LM, Martinbiancho JK, Andreolio C, da Rocha TS, Antonacci Carvalho PR, et al. Vancomycin-associated nephrotoxicity and risk factors in critically Ill children without preexisting renal injury. Pediatr Infect Dis J. 2019;38(9):934-8. http://doi.org/10.1097/INF.0000000000002391. PMid:31232892.
19. Chuphan C, Sukarnjanaset W, Puthanakit T, Wattanavijitkul T. Population pharmacokinetics and pharmacodynamics of vancomycin in pediatric patients with various degrees of renal function. J Pediatr Pharmacol Ther. 2022;27(5):419-27. http://doi.org/10.5863/1551-6776-27.5.419. PMid:35845555.
20. Le J, Ny P, Capparelli E, Lane J, Ngu B, Muus R, et al. Pharmacodynamic characteristics of nephrotoxicity associated with vancomycin use in children. J Pediatric Infect Dis Soc. 2015;4(4):e109-16. http://doi.org/10.1093/jpids/piu110. PMid:26582878.
21. Hill LF, Clements MN, Turner MA, Donà D, Lutsar I, Jacqz-Aigrain E, et al. Optimised versus standard dosing of vancomycin in infants with Gram-positive sepsis (NeoVanc): a multicentre, randomised, open-label, phase 2b, non-inferiority trial. Lancet Child Adolesc Health. 2022;6(1):49-59. http://doi.org/10.1016/S2352-4642(21)00305-9. PMid:34843669.
22. Williams C, Hankinson C, McWilliam SJ, Oni L. Vancomycin-associated acute kidney injury epidemiology in children: a systematic review. Arch Dis Child. 2022;107(10):947. http://doi.org/10.1136/archdischild-2021-323429. PMid:35210220.
23. Ragab AR, Al-Mazroua MK, Al-Harony MA. Incidence and predisposing factors of vancomycin-induced nephrotoxicity in children. Infect Dis Ther. 2013;2(1):37-46. http://doi.org/10.1007/s40121-013-0004-8. PMid:25135822.
24. Alzahrani AM, Naeem A, Alzhrani RM, Harbi MA, Alghamdi SA, Karim S, et al. The Bayesian-based area under the curve of vancomycin by using a single trough level: an evaluation of accuracy and discordance at tertiary care hospital in KSA. Healthcare. 2023;11(3):362. http://doi.org/10.3390/healthcare11030362. PMid:36766937.
25. Horey A, Mergenhagen KA, Mattappallil A. The Relationship of nephrotoxicity to vancomycin trough serum concentrations in a veteran’s population: a retrospective analysis. Ann Pharmacother. 2012;46(11):1477-83. http://doi.org/10.1345/aph.1R158. PMid:23073306.
26. Meaney CJ, Hynicka LM, Tsoukleris MG. Vancomycin-associated nephrotoxicity in adult medicine patients: incidence, outcomes, and risk factors. Pharmacotherapy. 2014;34(7):653-61. http://doi.org/10.1002/phar.1423. PMid:24700598.
27. Maung NH, Methaneethorn J, Wattanavijitkul T, Sriboonruang T. Comparison of area under the curve for vancomycin from one-and two-compartment models using sparse data. Eur J Hosp Pharm Sci Pract. 2022;29(e1):e57-62. http://doi.org/10.1136/ejhpharm-2020-002637. PMid:34285111.
28. Akunne OO, Mugabo P, Argent AC. Pharmacokinetics of vancomycin in critically ill children: a systematic review. Eur J Drug Metab Pharmacokinet. 2022;47(1):31-48. http://doi.org/10.1007/s13318-021-00730-z. PMid:34750740.
29. Silveira ALO, Alves GCDS, Xie J, Roberts JA, Sanches C. Vancomycin population pharmacokinetic modeling in children using Bayesian estimation and a Non Parametric. Braz J Pharm Sci. 2022;58:e19313. http://doi.org/10.1590/s2175-97902020000x2e19313.
30. Oliver MB, Boeser KD, Carlson MK, Hansen LA. Considerations for implementation of vancomycin Bayesian software monitoring in a level IV NICU population within a multisite health system. Am J Health Syst Pharm. 2023;80(11):670-7. http://doi.org/10.1093/ajhp/zxad048. PMid:36860169.