A associação entre concentrações no vale de vancomicina e nefrotoxicidade em uma unidade de terapia intensiva pediátrica
Conteúdo do artigo principal
Resumo
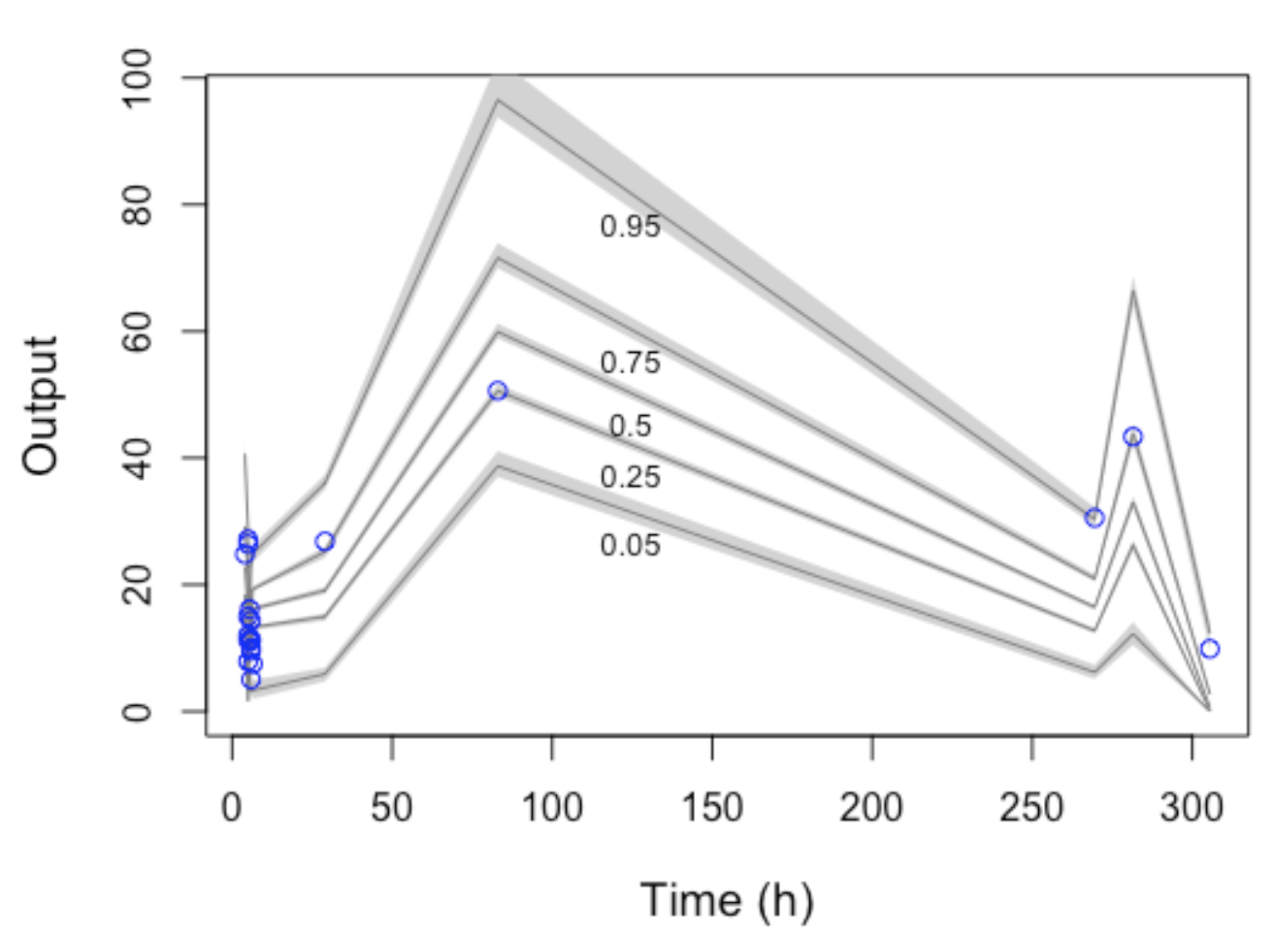
Objetivo: Analisar e descrever os aspectos farmacocinéticos do uso de vancomicina em uma coorte de crianças sob cuidados intensivos e elaborar um modelo farmacocinético para essa população. Método: Estudo observacional em uma unidade de terapia intensiva pediátrica conduzido entre setembro de 2017 a março de 2019. Inclui-se crianças em uso de vancomicina com pelo menos uma mensuração sérica desse antimicrobiano. As variáveis com valor de p < 0,2 na análise univariada e com plausibilidade biológica para propiciar nefrotoxicidade, não correlacionadas com outras preditoras, foram incluídas na regressão logística. Adicionalmente, uma modelagem farmacocinética foi realizada usando o programa PMETRICS® para pacientes com clearance de creatinina (CLCR) > 30 mL/min. Resultado: Foram incluídas 70 crianças no estudo. A dose média de vancomicina foi de 60 mg/kg/dia. Apenas onze crianças apresentaram vancocinemia dentro da faixa alvo (15-20 mg/L). Não foram observadas diferenças significativas entre as doses administradas e a observação de vancocinemia acima, dentro ou abaixo da faixa preconizada. No modelo multivariado, crianças acima da faixa sérica preconizada apresentaram odd ratio de 4,6 [IC 95% 1,4 – 17,2] para nefrotoxicidade. Um modelo farmacocinético com os dados de 15 crianças foi proposto, no qual os parâmetros de PK estimados para CLCR e Volume de distribuição foram de 0,94 L/h e 5,71 L, respectivamente. Conclusão: A nefrotoxicidade mostrou-se associada às concentrações plasmáticas de vancomicina iguais ou maiores a 15 mg/L. O modelo desenvolvido permitiu entender o comportamento do fármaco nessa população e pode ser útil na prática clínica para o monitoramento do uso de vancomicina.
Detalhes do artigo
Os autores mantêm os direitos autorais e concedem ao HSJ o direito de primeira publicação. A partir de 2024, as publicações serão licenciadas sob a Attribution 4.0 International 
 , permitindo seu compartilhamento, reconhecendo a autoria e publicação inicial nesta revista.
, permitindo seu compartilhamento, reconhecendo a autoria e publicação inicial nesta revista.
Os autores estão autorizados a assumir contratos adicionais separadamente para distribuição não exclusiva da versão do trabalho publicada nesta revista (por exemplo, publicação em repositório institucional ou como capítulo de livro), com reconhecimento de autoria e publicação inicial nesta revista.
Os autores são incentivados a publicar e distribuir seu trabalho on-line (por exemplo, em repositórios institucionais ou em sua página pessoal) a qualquer momento após o processo editorial.
Além disso, o AUTOR fica informado e consente que o HSJ possa incorporar seu artigo em bases de dados e indexadores científicos existentes ou futuros, nas condições definidas por estes a cada momento, o que envolverá, pelo menos, a possibilidade de que os titulares de esses bancos de dados podem executar as seguintes ações no artigo.
Referências
Porto APM, Goossens H, Versporten A, Costa SF. Global point prevalence survey of antimicrobial consumption in Brazilian hospitals. J Hosp Infect. 2020;104(2):165-71. http://doi.org/10.1016/j.jhin.2019.10.016. PMid:31678430. DOI: https://doi.org/10.1016/j.jhin.2019.10.016
Araujo da Silva AR, Jaszkowski E, Schober T, von Both U, Meyer-Buehn M, Marques AF, et al. Patterns of antimicrobial consumption in neonatal and pediatric intensive care units in Germany and Brazil. Eur J Clin Microbiol Infect Dis. 2020;39(2):249-55. http://doi.org/10.1007/s10096-019-03714-9. PMid:31673879. DOI: https://doi.org/10.1007/s10096-019-03714-9
Sridharan K, Abbasi MY, Mulubwa M. Population pharmacokinetics and dose optimization of vancomycin in critically Ill children. Eur J Drug Metab Pharmacokinet. 2021;46(4):539-46. http://doi.org/10.1007/s13318-021-00695-z. PMid:34156647. DOI: https://doi.org/10.1007/s13318-021-00695-z
Cao L, Li Z, Zhang P, Yong S. Relationship between vancomycin trough serum concentrations and clinical outcomes in children: a systematic review and meta-analysis. Antimicrob Agents Chemother. 2022;66(8):e00138-22. http://doi.org/10.1128/aac.00138-22. PMid:35862741. DOI: https://doi.org/10.1128/aac.00138-22
He N, Su S, Ye Z, Du G, He B, Li D, et al. Evidence-based Guideline for Therapeutic Drug Monitoring of Vancomycin: 2020 Update by the Division of Therapeutic Drug Monitoring, Chinese Pharmacological Society. Clin Infect Dis. 2020;71(Suppl 4):S363- 71. http://doi.org/10.1093/cid/ciaa1536. PMid:33367582. DOI: https://doi.org/10.1093/cid/ciaa1536
Filippone EJ, Kraft WK, Farber JL. The nephrotoxicity of vancomycin. Clin Pharmacol Ther. 2017;102(3):459-69. http:// doi.org/10.1002/cpt.726. PMid:28474732. DOI: https://doi.org/10.1002/cpt.726
Shah S, Barton G, Fischer A. Pharmacokinetic considerations and dosing strategies of antibiotics in the critically ill patient. J Intensive Care Soc. 2015;16(2):147-53. http://doi.org/10.1177/1751143714564816. PMid:28979397. DOI: https://doi.org/10.1177/1751143714564816
McNeil JC, Kaplan SL. Vancomycin therapeutic drug monitoring in children: new recommendations, similar challenges. J Pediatr Pharmacol Ther. 2020;25(6):472-5. http://doi.org/10.5863/1551-6776-25.6.472. PMid:32839650. DOI: https://doi.org/10.5863/1551-6776-25.6.472
Lee BV, Fong G, Bolaris M, Neely M, Minejima E, Kang A, et al. Cost-benefit analysis comparing trough, two-level AUC and Bayesian AUC dosing for vancomycin. Clin Microbiol Infect. 2021;27(9):1346.e1-7. http://doi.org/10.1016/j.cmi.2020.11.008. PMid:33221430. DOI: https://doi.org/10.1016/j.cmi.2020.11.008
Tsutsuura M, Moriyama H, Kojima N, Mizukami Y, Tashiro S, Osa S, et al. The monitoring of vancomycin: a systematic review and meta-analyses of area under the concentration-time curve-guided dosing and trough-guided dosing. BMC Infect Dis. 2021;21(1):153. http://doi.org/10.1186/s12879-021-05858-6. PMid:33549035. DOI: https://doi.org/10.1186/s12879-021-05858-6
Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835- 64. http://doi.org/10.1093/ajhp/zxaa036. PMid:32191793. DOI: https://doi.org/10.1093/ajhp/zxaa036
Zamoner W, Prado IRS, Balbi AL, Ponce D. Vancomycin dosing, monitoring and toxicity: Critical review of the clinical practice. Clin Exp Pharmacol Physiol. 2019;46(4):292-301. http://doi.org/10.1111/1440-1681.13066. PMid:30623980. DOI: https://doi.org/10.1111/1440-1681.13066
Chung E, Sen J, Patel P, Seto W. Population pharmacokinetic models of vancomycin in paediatric patients: a systematic review. Clin Pharmacokinet. 2021;60(8):985-1001. http://doi.org/10.1007/s40262-021-01027-9. PMid:34002357. DOI: https://doi.org/10.1007/s40262-021-01027-9
World Health Organization [Internet]. Geneva: WHO; 2023 [cited 2023 Aug 7]. Available from: https://www.who.int
Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering RC Jr, Craig WA, Billeter M, et al. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the Infectious Diseases Society of America, the American Society of Health- System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis. 2009;49(3):325-7. http://doi.org/10.1086/600877. PMid:19569969. DOI: https://doi.org/10.1086/600877
UpToDate. Lexicomp® Drug Interactions [Internet]. 2023 [cited 2023 Aug 10]. Available from: https://www.uptodate.com/drug-interactions/?source=responsive_home#di-druglist DOI: https://doi.org/10.1097/01.NPR.0000000000000019
Laboratory of Applied Pharmacokinetics and Bioinformatics. Pmetrics [Internet]. 2023 [cited 2023 Aug 10]. Available from: https://www.lapk.org/software.php
Feiten HDS, Okumura LM, Martinbiancho JK, Andreolio C, da Rocha TS, Antonacci Carvalho PR, et al. Vancomycin-associated nephrotoxicity and risk factors in critically Ill children without preexisting renal injury. Pediatr Infect Dis J. 2019;38(9):934-8. http://doi.org/10.1097/INF.0000000000002391. PMid:31232892. DOI: https://doi.org/10.1097/INF.0000000000002391
Chuphan C, Sukarnjanaset W, Puthanakit T, Wattanavijitkul T. Population pharmacokinetics and pharmacodynamics of vancomycin in pediatric patients with various degrees of renal function. J Pediatr Pharmacol Ther. 2022;27(5):419-27. http://doi.org/10.5863/1551-6776-27.5.419. PMid:35845555. DOI: https://doi.org/10.5863/1551-6776-27.5.419
Le J, Ny P, Capparelli E, Lane J, Ngu B, Muus R, et al. Pharmacodynamic characteristics of nephrotoxicity associated with vancomycin use in children. J Pediatric Infect Dis Soc. 2015;4(4):e109-16. http://doi.org/10.1093/jpids/piu110. PMid:26582878. DOI: https://doi.org/10.1093/jpids/piu110
Hill LF, Clements MN, Turner MA, Donà D, Lutsar I, Jacqz-Aigrain E, et al. Optimised versus standard dosing of vancomycin in infants with Gram-positive sepsis (NeoVanc): a multicentre, randomised, open-label, phase 2b, non-inferiority trial. Lancet Child Adolesc Health. 2022;6(1):49-59. http://doi.org/10.1016/S2352-4642(21)00305-9. PMid:34843669. DOI: https://doi.org/10.1016/S2352-4642(21)00305-9
Williams C, Hankinson C, McWilliam SJ, Oni L. Vancomycin-associated acute kidney injury epidemiology in children: a systematic review. Arch Dis Child. 2022;107(10):947. http://doi.org/10.1136/archdischild-2021-323429. PMid:35210220. DOI: https://doi.org/10.1136/archdischild-2021-323429
Ragab AR, Al-Mazroua MK, Al-Harony MA. Incidence and predisposing factors of vancomycin-induced nephrotoxicity in children. Infect Dis Ther. 2013;2(1):37-46. http://doi.org/10.1007/s40121-013-0004-8. PMid:25135822. DOI: https://doi.org/10.1007/s40121-013-0004-8
Alzahrani AM, Naeem A, Alzhrani RM, Harbi MA, Alghamdi SA, Karim S, et al. The Bayesian-based area under the curve of vancomycin by using a single trough level: an evaluation of accuracy and discordance at tertiary care hospital in KSA. Healthcare. 2023;11(3):362. http://doi.org/10.3390/healthcare11030362. PMid:36766937. DOI: https://doi.org/10.3390/healthcare11030362
Horey A, Mergenhagen KA, Mattappallil A. The Relationship of nephrotoxicity to vancomycin trough serum concentrations in a veteran’s population: a retrospective analysis. Ann Pharmacother. 2012;46(11):1477-83. http://doi.org/10.1345/aph.1R158. PMid:23073306. DOI: https://doi.org/10.1345/aph.1R158
Meaney CJ, Hynicka LM, Tsoukleris MG. Vancomycin-associated nephrotoxicity in adult medicine patients: incidence, outcomes, and risk factors. Pharmacotherapy. 2014;34(7):653-61. http://doi.org/10.1002/phar.1423. PMid:24700598. DOI: https://doi.org/10.1002/phar.1423
Maung NH, Methaneethorn J, Wattanavijitkul T, Sriboonruang T. Comparison of area under the curve for vancomycin from one-and two-compartment models using sparse data. Eur J Hosp Pharm Sci Pract. 2022;29(e1):e57-62. http://doi.org/10.1136/ejhpharm-2020-002637. PMid:34285111. DOI: https://doi.org/10.1136/ejhpharm-2020-002637
Akunne OO, Mugabo P, Argent AC. Pharmacokinetics of vancomycin in critically ill children: a systematic review. Eur J Drug Metab Pharmacokinet. 2022;47(1):31-48. http://doi.org/10.1007/s13318-021-00730-z. PMid:34750740. DOI: https://doi.org/10.1007/s13318-021-00730-z
Silveira ALO, Alves GCDS, Xie J, Roberts JA, Sanches C. Vancomycin population pharmacokinetic modeling in children using Bayesian estimation and a Non Parametric. Braz J Pharm Sci. 2022;58:e19313. http://doi.org/10.1590/s2175-97902020000x2e19313. DOI: https://doi.org/10.1590/s2175-97902020000x2e19313
Oliver MB, Boeser KD, Carlson MK, Hansen LA. Considerations for implementation of vancomycin Bayesian software monitoring in a level IV NICU population within a multisite health system. Am J Health Syst Pharm. 2023;80(11):670-7. http://doi.org/10.1093/ajhp/zxad048. PMid:36860169. DOI: https://doi.org/10.1093/ajhp/zxad048