Alterações osteometabólicas em pacientes em tratamento antineoplásico: revisão de escopo
Conteúdo do artigo principal
Resumo
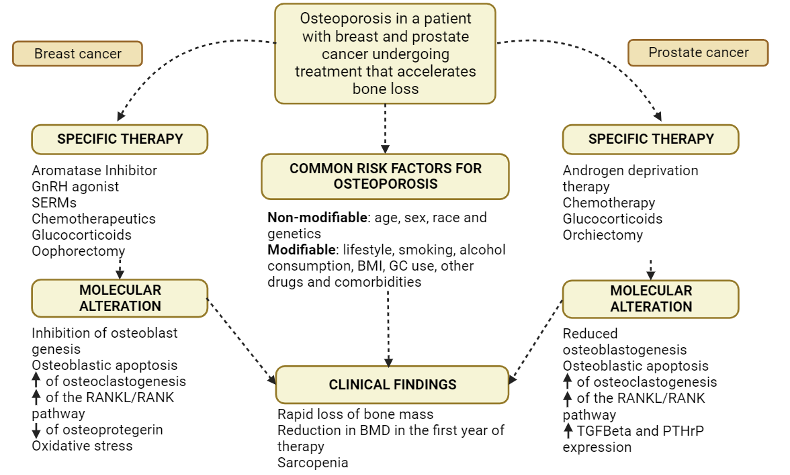
Objetivo: sintetizar as principais evidências acerca das alterações osteometabólicas presentes nos pacientes em tratamento antineoplásico. Métodos: trata-se de uma revisão de escopo, seguindo a metodologia do Instituto Joanna Briggs, nas bases de dados PubMed/MedLine, Cochrane Library, LILACS, The British Library e Google Scholar. A revisão está protocolada no Open Science Framework. Resultados: muitos antineoplásicos possuem efeito na arquitetura óssea, reduzindo sua densidade, tais como moduladores seletivos de receptores de estrogênio, inibidores da aromatase, terapia de privação androgênica, e glicocorticoides. Para evitar tais desfechos, o tratamento e prevenção podem ser realizados pela suplementação de cálcio e vitamina D, exercícios físicos, uso de bifosfonatos, denosumab, e moduladores seletivos do receptor de estrogênio. Conclusão: pessoas com maior risco de desenvolver câncer também possuem maior risco de osteopenia e osteoporose, quando processo já estabelecido e em tratamento antineoplásico, devido ao compartilhamento de fatores de risco. Torna-se evidente a necessidade da densitometria óssea nos pacientes em tratamento contra o câncer para de prevenção e promoção de saúde óssea nesses pacientes, além de mais pesquisas com alto nivel de evidência para subsidiar tal uso.
Detalhes do artigo
Os autores mantêm os direitos autorais e concedem ao HSJ o direito de primeira publicação. A partir de 2024, as publicações serão licenciadas sob a Attribution 4.0 International 
 , permitindo seu compartilhamento, reconhecendo a autoria e publicação inicial nesta revista.
, permitindo seu compartilhamento, reconhecendo a autoria e publicação inicial nesta revista.
Os autores estão autorizados a assumir contratos adicionais separadamente para distribuição não exclusiva da versão do trabalho publicada nesta revista (por exemplo, publicação em repositório institucional ou como capítulo de livro), com reconhecimento de autoria e publicação inicial nesta revista.
Os autores são incentivados a publicar e distribuir seu trabalho on-line (por exemplo, em repositórios institucionais ou em sua página pessoal) a qualquer momento após o processo editorial.
Além disso, o AUTOR fica informado e consente que o HSJ possa incorporar seu artigo em bases de dados e indexadores científicos existentes ou futuros, nas condições definidas por estes a cada momento, o que envolverá, pelo menos, a possibilidade de que os titulares de esses bancos de dados podem executar as seguintes ações no artigo.
Referências
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Can Clin J. 2021;71(3):209-49. https://doi.org/10.3322/caac.21660 DOI: https://doi.org/10.3322/caac.21660
Bedatsova L, Drake MT. The skeletal impact of cancer therapies. Br J Clin Pharmacol. 2019;85(6):1161-8. https://doi.org/10.1111/bcp.13866 DOI: https://doi.org/10.1111/bcp.13866
Reuss-Borst M, Hartmann U, Scheede C, Weiß J. Prevalence of osteoporosis among cancer patients in Germany. Osteoporos Int. 2011;23(4):1437-44. https://doi.org/10.1007/s00198-011-1724-9 DOI: https://doi.org/10.1007/s00198-011-1724-9
Park SH, Knobf MT, Sutton KM. Etiology, assessment, and management of aromatase inhibitor-related musculoskeletal symptoms. Clin J Oncol Nurs. 2012;16(3):260-6. https://doi.org/10.1188/12.CJON.260-266 DOI: https://doi.org/10.1188/12.CJON.260-266
Van Poznak C, Somerfield MR, Barlow WE et al. Role of bone-modifying agents in metastatic breast cancer: an american society of clinical oncology-cancer care ontario focused guideline update. J Clin Oncol. 2017;35(35):3978-86. https://doi.org/10.1200/JCO.2017.75.4614 DOI: https://doi.org/10.1200/JCO.2017.75.4614
Rachner TD, Coleman R, Hadji P, Hofbauer LC. Bone health during endocrine therapy for cancer. Lancet Diabetes Amp Endocrinol. 2018;6(11):901-10. https://doi.org/10.1016/S2213-8587(18)30047-0 DOI: https://doi.org/10.1016/S2213-8587(18)30047-0
Morgan RL, Whaley P, Thayer KA, Schünemann HJ. Identifying the PECO: a framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ Int. 2018;121:1027-31. https://doi.org/10.1016/j.envint.2018.07.015 DOI: https://doi.org/10.1016/j.envint.2018.07.015
Melnyk BM, Ellen Fineout-Overholt E. Evidence-Based Practice in Nursing & Healthcare: A Guide to Best Practice (4th ed). LWW: Philadelphia, PA; 2018.
Lopes-Júnior LC, Bomfim E, Olson K, et al. Effectiveness of hospital clowns for symptom management in paediatrics: systematic review of randomised and non-randomised controlled trials. BMJ. 2020;371:m4290. https://doi.org/10.1136/bmj.m4290 DOI: https://doi.org/10.1136/bmj.m4290
Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (Prisma-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467. https://doi.org/10.7326/M18-0850 DOI: https://doi.org/10.7326/M18-0850
Lopes-Júnior LC, Rosa MA, Lima RA. Psychological and psychiatric outcomes following PICU admission. Pediatr Crit Care Med. 2018;19(1):e58-67. https://doi.org/10.1097/PCC.0000000000001390 DOI: https://doi.org/10.1097/PCC.0000000000001390
Casemiro LK, Lopes-Júnior LC, Jardim FA, Sulino MC, de Lima RA. Telehealth in outpatient care for children and adolescents with chronic conditions during the COVID-19 pandemic: a scoping review protocol. PLoS One. 2022;17(6):e0269821 https://doi.org/10.1371/journal.pone.0269821 DOI: https://doi.org/10.1371/journal.pone.0269821
Law M. Guideline for Critical Review Form - Quantitative Studies. McMaster University Occupational Therapy Evidence-Based Practice Research Group; Canadá.1998. Available from: https://bit.ly/3EzQm0O DOI: https://doi.org/10.1177/000841749806500301
El Badri S, Salawu A, Brown JE. Bone health in men with prostate cancer: review article. Curr Osteoporos Rep. 2019;17(6):527-37. https://doi.org/10.1007/s11914-019-00536-8 DOI: https://doi.org/10.1007/s11914-019-00536-8
Liuhto N, Grönroos MH, Malila N, Madanat‐Harjuoja L, Matomäki J, Lähteenmäki P. Diseases of renal function and bone metabolism after treatment for early onset cancer: a registry‐based study. Int J Cancer. 2019;146(5):1324-32. https://doi.org/10.1002/ijc.32687 DOI: https://doi.org/10.1002/ijc.32687
Owen PJ, Daly RM, Livingston PM, Fraser SF. Lifestyle guidelines for managing adverse effects on bone health and body composition in men treated with androgen deprivation therapy for prostate cancer: an update. Prostate Cancer Prostatic Dis. 2017;20(2):137-45. https://doi.org/10.1038/pcan.2016.69 DOI: https://doi.org/10.1038/pcan.2016.69
Schyrr F, Wolfer A, Pasquier J, Nicoulaz AL, Lamy O, Naveiras O. Correlation study between osteoporosis and hematopoiesis in the context of adjuvant chemotherapy for breast cancer. Ann Hematol. 2017;97(2):309-17. https://doi.org/10.1007/s00277-017-3184-6
van Hellemond IEG, Smorenburg CH, Peer PGM, Swinkels ACP, Seynaeve CM, van der Sangen MJC, et al. Breast cancer outcome in relation to bone mineral density and bisphosphonate use: a sub-study of the DATA trial. Breast Cancer Res Treat. 2020;180(3):675-85. https://doi.org/10.1007/s10549-020-05567-9 DOI: https://doi.org/10.1007/s10549-020-05567-9
Seland M, Smeland KB, Bjøro T, Falk RS, Fosså SD, Gjesdal CG, et al. Bone mineral density is close to normal for age in long-term lymphoma survivors treated with high-dose therapy with autologous stem cell transplantation. Acta Oncol. 2017;56(4):590-8. https://doi.org/10.1080/0284186X.2016.1267870 DOI: https://doi.org/10.1080/0284186X.2016.1267870
Sestak I, Blake GM, Patel R, Coleman RE, Cuzick J, Eastell R. Comparison of risedronate versus placebo in preventing anastrozole-induced bone loss in women at high risk of developing breast cancer with osteopenia. Bone. 2019;124:83-8. https://doi.org/10.1016/j.bone.2019.04.016 DOI: https://doi.org/10.1016/j.bone.2019.04.016
Castañeda S, Casas A, González-Del-Alba A, Martínez-Díaz-Guerra G, Nogués X, Ojeda Thies C, et al. Bone loss induced by cancer treatments in breast and prostate cancer patients. Clin Transl Oncol. 2022;24(11):2090-106. https://doi.org/10.1007/s12094-022-02872-1 DOI: https://doi.org/10.1007/s12094-022-02872-1
Majithia N, Atherton PJ, Lafky JM, Wagner-Johnston N, Olson J, et al. Zoledronic acid for treatment of osteopenia and osteoporosis in women with primary breast cancer undergoing adjuvant aromatase inhibitor therapy: a 5-year follow-up. Support Care Cancer. 2015;24(3):1219-26. https://doi.org/10.1007/s00520-015-2915-2 DOI: https://doi.org/10.1007/s00520-015-2915-2
Livi L, Scotti V, Desideri I, Saieva C, Cecchini S, Francolini G, et al. Phase 2 placebo-controlled, single-blind trial to evaluate the impact of oral ibandronate on bone mineral density in osteopenic breast cancer patients receiving adjuvant aromatase inhibitors: 5-year results of the single-centre BONADIUV trial. Eur J Cancer. 2019;108:100-110. https://doi.org/10.1016/j.ejca.2018.12.005 DOI: https://doi.org/10.1016/j.ejca.2018.12.005
Harvey NC, Glüer CC, Binkley N et al. Trabecular bone score (TBS) as a new complementary approach for osteoporosis evaluation in clinical practice. Bone. 2015;78:216-24. https://doi.org/10.1016/j.bone.2015.05.016 DOI: https://doi.org/10.1016/j.bone.2015.05.016
Lane NE. Glucocorticoid-Induced osteoporosis: new insights into the pathophysiology and treatments. Curr Osteoporos Rep. 2019;17(1):1-7. https://doi.org/10.1007/s11914-019-00498-x DOI: https://doi.org/10.1007/s11914-019-00498-x
Smith MR, Fallon MA, Lee H, Finkelstein JS. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Amp Metab. 2004;89(8):3841-6. https://doi.org/10.1210/jc.2003-032058 DOI: https://doi.org/10.1210/jc.2003-032058
Gao Q, López-Knowles E, Cheang MC et al. Impact of aromatase inhibitor treatment on global gene expression and its association with antiproliferative response in ER+ breast cancer in postmenopausal patients. Breast Cancer Res. 2019;22(1):2. https://doi.org/10.1186/s13058-019-1223-z DOI: https://doi.org/10.1186/s13058-019-1223-z
Schyrr F, Wolfer A, Pasquier J, Nicoulaz AL, Lamy O, Naveiras O. Correlation study between osteoporosis and hematopoiesis in the context of adjuvant chemotherapy for breast cancer. Ann Hematol. 2017;97(2):309-17. https://doi.org/10.1007/s00277-017-3184-6 DOI: https://doi.org/10.1007/s00277-017-3184-6
Paterson AH, Anderson SJ, Lembersky BC et al. Oral clodronate for adjuvant treatment of operable breast cancer (National Surgical Adjuvant Breast and Bowel Project protocol B-34): a multicentre, placebo-controlled, randomised trial. Lancet Oncol. 2012;13(7):734-42. https://doi.org/10.1016/S1470-2045(12)70226-7 DOI: https://doi.org/10.1016/S1470-2045(12)70226-7
EBCTCG (Early Breast Cancer Trialists' Collaborative Group). Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127-35. https://doi.org/10.1016/S0140-6736(14)60488-8 DOI: https://doi.org/10.1016/S0140-6736(14)60488-8
Denham JW, Joseph D, Lamb DS, Spry NA, Duchesne G, Matthews J, et al. Short-term androgen suppression and radiotherapy versus intermediate-term androgen suppression and radiotherapy, with or without zoledronic acid, in men with locally advanced prostate cancer (TROG 03.04 RADAR): 10-year results from a randomised, phase 3, factorial trial. Lancet Oncol. 2019;20(2):267-81. https://doi.org/10.1016/S1470-2045(18)30757-5 DOI: https://doi.org/10.1016/S1470-2045(18)30757-5
Clarke BL. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. Yearb Med. 2010;2010:487-9. https://doi.org/10.1016/S0084-3873(10)79797-2 DOI: https://doi.org/10.1016/S0084-3873(10)79797-2