Association of great saphenous vein diameter and clinical severity score after treatment of severe chronic venous insufficiency with foam sclerotherapy
Main Article Content
Abstract
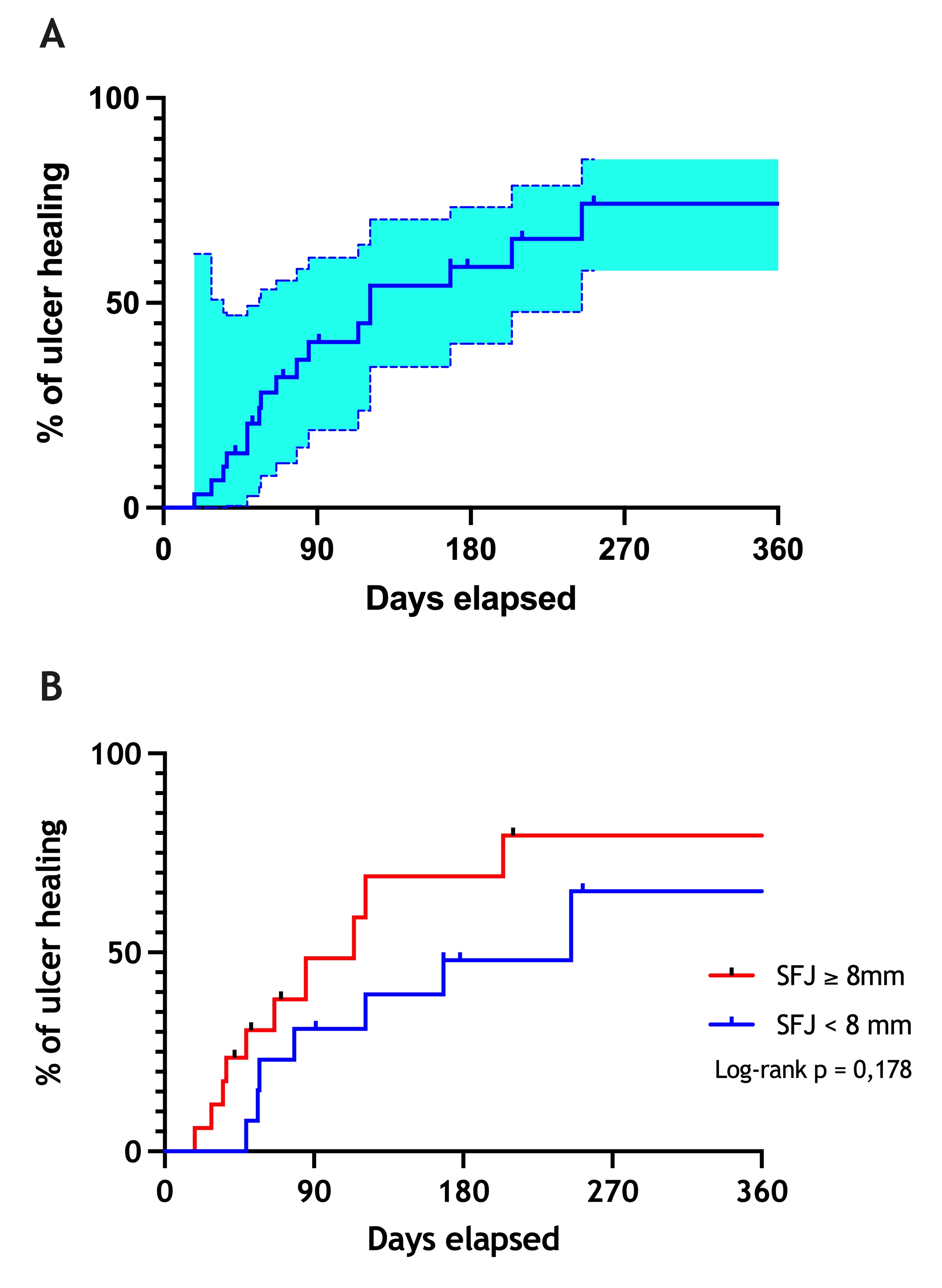
Objectives: to evaluate the association of the greater saphenous vein (GSV) diameter in the treatment of patients with severe chronic venous insufficiency (C6 CEAP classification) with ultrasound-guided polidocanol foam sclerotherapy (UGFS). Methods: A prospective, descriptive and analytical study of 28 patients (30 limbs) that underwent UGFS. Patients were divided into 2 subgroups by GSV diameter (< 8 mm and ≥ 8 mm). Variables analyzed were ulcer healing, clinical intercurrences, clinical CEAP classification, Venous Clinical Severity Score (VCSS), diameter of the treated vein and presence of occlusion or recanalization by Doppler ultrasound. Patients were analyzed at the 1st, 3rd, and 6th months post-treatment. Results: The average age was 68.7 ± 10.5 years, 23 (82,1%) were women, and the average body mass index was 29.2 kg/m2. Although an improvement in VCSS score was observed during follow-up, no significant intergroup difference was noted. Seventeen (56%) limbs presented occlusion of the treated vein at the 1st month, 11 (36%) at the 3rd month, and 9 (30%) at the 6th month of follow-up. The ulcer healing rate was 56,6%. The average ulcer healing time was 90 days. Three (10%) patients presented with ulcer recurrence at the 6th month. Survival analysis showed no significant difference in ulcer healing rate between subgroups after one year of follow-up (log-rank, p = 0,178). Conclusion: There was no difference between the subgroups of large and small VSM diameter in terms of symptom severity. However, significant reduction of VCSS and pain relief was observed after foam sclerotherapy.
Article Details
Authors maintain copyright and grant the HSJ the right to first publication. From 2024, the publications wiil be licensed under Attribution 4.0 International 
 , allowing their sharing, recognizing the authorship and initial publication in this journal.
, allowing their sharing, recognizing the authorship and initial publication in this journal.
Authors are authorized to assume additional contracts separately for the non-exclusive distribution of the version of the work published in this journal (e.g., publishing in an institutional repository or as a book chapter), with acknowledgment of authorship and initial publication in this journal.
Authors are encouraged to publish and distribute their work online (e.g., in institutional repositories or on their personal page) at any point after the editorial process.
Also, the AUTHOR is informed and consents that the HSJ can incorporate his article into existing or future scientific databases and indexers, under the conditions defined by the latter at all times, which will involve, at least, the possibility that the holders of these databases can perform the following actions on the article.
References
Mansilha A, Sousa J. Pathophysiological mechanisms of chronic venous disease and implications for venoactive drug therapy. Int J Mol Sci. 2018;19(6):1669. https://doi.org/10.3390/ijms19061669
Bergan JJ, Schmid-Schönbein GW, Smith PD, Nicolaides AN, Boisseau MR, Eklof B. Chronic venous disease. N Engl J Med. 2006;355(5):488-98. https://doi.org/10.1056/NEJMra055289
Raffetto J, Eberhardt RT. Chronic venous disorders: general considerations. In: Cronenwett JL, Johnston KW, eds. Rutheford's Textbook of Vascular Surgery, 7th Edition. Philadelphia, PA: Saunders-Elsevier; 2010. p. 831-843
Kaplan RM, Criqui MH, Denenberg JO, Bergan J, Fronek A. Quality of life in patients with chronic venous disease: San Diego population study. J Vasc Surg. 2003;37(5):1047-53. https://doi.org/10.1067/mva.2003.168
Eberhardt RT, Raffetto JD. Chronic venous insufficiency. Circulation. 2014;130(4):333-46. https://doi.org/10.1161/CIRCULATIONAHA.113.006898
Alder G, Lees T. Foam sclerotherapy. Phlebology. 2015;30(2 Suppl):18-23. https://doi.org/10.1177/0268355515589536
Silva MAM, Araujo ÁZP, do Amaral JF, Jesus-Silva SG, Cardoso RS, Miranda F Jr. Impacto da escleroterapia com espuma de polidocanol guiada por ultrassom em pacientes com úlcera venosa. J Vasc Bras. 2017;16(3):239-43. https://doi.org/10.1590/1677-5449.002717
Shadid N, Ceulen R, Nelemans P, et al. Randomized clinical trial of ultrasound-guided foam sclerotherapy versus surgery for the incompetent great saphenous vein. Br J Surg. 2012;99(8):1062-70. https://doi.org/10.1002/bjs.8781
Jia X, Mowatt G, Burr JM, Cassar K, Cook J, Fraser C. Systematic review of foam sclerotherapy for varicose veins. Br J Surg. 2007;94(8):925-36 https://doi.org/10.1002/bjs.5891
Tessari L, Cavezzi A, Frullini A. Preliminary experience with a new sclerosing foam in the treatment of varicose veins. Dermatol Surg. 2001;27(1):58-60. https://doi.org/10.1097/00042728-200101000-00017
Yamaki T, Hamahata A, Soejima K, Kono T, Nozaki M, Sakurai H. Prospective randomised comparative study of visual foam sclerotherapy alone or in combination with ultrasound-guided foam sclerotherapy for treatment of superficial venous insufficiency: preliminary report. Eur J Vasc Endovasc Surg. 2012;43(3):343-7. https://doi.org/10.1016/j.ejvs.2011.07.029
Silva MAM, Burihan MC, Barros OC, Nasser F, Ingrund JC, Neser A. Resultados do tratamento da Insuficiência Venosa Crônica grave com espuma de polidocanol guiada por ultrassom. J Vasc Bras. 2012;11(3):206-11. https://doi.org/10.1590/S1677-54492012000300007
Star P, Connor DE, Parsi K. Novel developments in foam sclerotherapy: Focus on Varithena® (polidocanol endovenous microfoam) in the management of varicose veins. Phlebology. 2018;33(3):150-62. https://doi.org/10.1177/0268355516687864
Carugo D, Ankrett DN, Zhao X, et al. Benefits of polidocanol endovenous microfoam (Varithena®) compared with physician-compounded foams. Phlebology. 2016;31(4):283-95. https://doi.org/10.1177/0268355515589063
Lawaetz M, Serup J, Lawaetz B, et al. Comparison of endovenous ablation techniques, foam sclerotherapy and surgical stripping for great saphenous varicose veins. Extended 5-year follow-up of a RCT. Int Angiol. 2017;36(3):281-8. https://doi.org/10.23736/S0392-9590.17.03827-5
Venermo M, Saarinen J, Eskelinen E, et al. Randomized clinical trial comparing surgery, endovenous laser ablation and ultrasound-guided foam sclerotherapy for the treatment of great saphenous varicose veins. Br J Surg. 2016;103(11):1438-44. https://doi.org/10.1002/bjs.10260
Campos W Jr, Torres IO, da Silva ES, Casella IB, Puech-Leão P. A prospective randomized study comparing polidocanol foam sclerotherapy with surgical treatment of patients with primary chronic venous insufficiency and ulcer. Ann Vasc Surg. 2015;29(6):1128-35. https://doi.org/10.1016/j.avsg.2015.01.031
Rasmussen LH, Lawaetz M, Bjoern L, Vennits B, Blemings A, Eklof B. Randomized clinical trial comparing endovenous laser ablation, radiofrequency ablation, foam sclerotherapy and surgical stripping for great saphenous varicose veins. Br J Surg. 2011;98(8):1079-87. https://doi.org/10.1002/bjs.7555
Myers KA, Jolley D, Clough A, et al. Outcome of ultrasound-guided sclerotherapy for varicose veins: mediumterm results assessed by ultrasound surveillance. Eur J Vasc Endovasc Surg 2007; 33:116-21. https://doi.org/10.1016/j.ejvs.2006.09.005
Shadid N, Nelemans P, Lawson J, Sommer A. Predictors of recurrence of great saphenous vein reflux following treatment with ultrasound-guided foam sclerotherapy. Phlebology. 2015;30(3):194-9. https://doi.org/10.1177/0268355514521183
Hamel-Desnos CM, De Maeseneer M, Josnin M, Gillet JL, Allaert FA; DIAGRAVES Study Group. Great Saphenous Vein Diameters in Phlebological Practice in France: A Report of the DIAGRAVES Study by the French Society of Phlebology. Eur J Vasc Endovasc Surg. 2019;58(1):96-103. https://doi.org/10.1016/j.ejvs.2018.09.011
Shaidakov EV, Grigoryan AG, Ilyukhin EA, Bulatov VL, Rosukhovskiy DA. Radiofrequency ablation or stripping of large-diameter incompetent great saphenous varicose veins with C2 or C3 disease. J Vasc Surg Venous Lymphat Disord. 2016;4(1):45-50. https://doi.org/10.1016/j.jvsv.2015.07.007
Woo HY, Kim SM, Kim D, Chung JK, Jung IM. Outcome of ClosureFAST radiofrequency ablation for large-diameter incompetent great saphenous vein. Ann Surg Treat Res. 2019;96(6):313-8. https://doi.org/10.4174/astr.2019.96.6.313
Eklöf B, Rutherford RB, Bergan JJ, et al. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg. 2004;40(6):1248-52. https://doi.org/10.1016/j.jvs.2004.09.027
Rutherford RB, Padberg FT Jr, Comerota AJ, Kistner RL, Meissner MH, Moneta GL. Venous severity scoring: An adjunct to venous outcome assessment. J Vasc Surg. 2000;31(6):1307-12. https://doi.org/10.1067/mva.2000.107094
Cabrera J, Redondo P, Becerra A, et al. Ultrasound-guided injection of polidocanol microfoam in the management of venous leg ulcers. Arch Dermatol. 2004;140(6):667-73. https://doi.org/10.1001/archderm.140.6.667
Attaran RR, Bhalla A, Mena-Hurtado CI, Ochoa Chaar CII. Correlation between great saphenous length of treatment zone and diameter with improvement in symptoms post-ablation. J Vasc Surg Venous Lymphat Disord. 2021;S2213-333X(21)00099-8. https://doi.org/10.1016/j.jvsv.2021.02.013
Abreu GCG, Camargo Jr. O, Abreu MFM, Aquino JLB. Ultrasound-guided foam sclerotherapy for chronic venous disease with ulcer. A prospective multiple outcome cohort study. J Vasc Bras. 2020;19:e20180108. https://doi.org/10.1590/1677-5449.180108
Coelho Neto F, Araújo GR, Kessler IM. Evaluation of quality of life and photoplethysmography in patients with chronic venous insufficiency treated with foam sclerotherapy. J Vasc Bras. 2015;14(2):145-52. https://doi.org/10.1590/1677-5449.8314
Howard JK, Slim FJ, Wakely MC, et al. Recanalisation and ulcer recurrence rates following ultrasound-guided foam sclerotherapy. Phlebology. 2016;31(7):506-13. https://doi.org/10.1177/0268355515598450
Scotton MF, Miot HA, Abbade LPF. Factors that influence healing of chronic venous leg ulcers: a retrospective cohort. An Bras Dermatol. 2014;89(3):414-22. https://doi.org/10.1590/abd1806-4841.20142687
Milic DJ, Zivic SS, Bogdanovic DC, Karanovic ND, Golubovic ZV. Risk factors related to the failure of venous leg ulcers to heal with compression treatment. J Vasc Surg. 2009;49(5):1242-7. https://doi.org/10.1016/j.jvs.2008.11.069